Understanding Electrolysis
13 min. read
Spontaneous Reactions
Redox
A neutral atom with atomic number contains (by definition) protons. In order to remain neutral, then, the atom must contain electrons. These electrons are organised under the quantum model into shells of discrete energy orbits around the nucleus. The outermost of these shells is denoted as the valence shell. If given enough energy, electrons can be liberated from their nucleus orbit. The least amount of energy is required to liberate electrons in the valence shell.
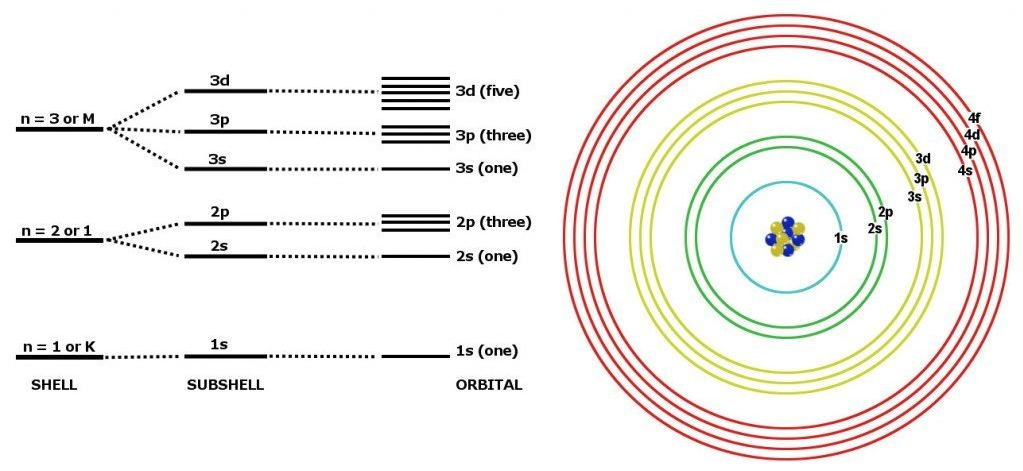
Each shell has a maximum capacity of electrons that it can carry, before a new shell needs to begin filling, and they fill from inwards to outwards. In general, the most stable configuration for an atom is to have its valence shell properly filled (that is, so it becomes neutral). So, if one atom requiring an electron to achieve stability comes near an atom that can donate an electron from its valence shell to achieve greater stability, a spontaneous reaction will take place. This is the basis of a system of reactions called redox reactions.
In redox reactions, one compound loses electrons, and the other compound gains electrons. The former is termed the reductant and it undergoes oxidation. The latter is termed the oxidant and undergoes reduction.
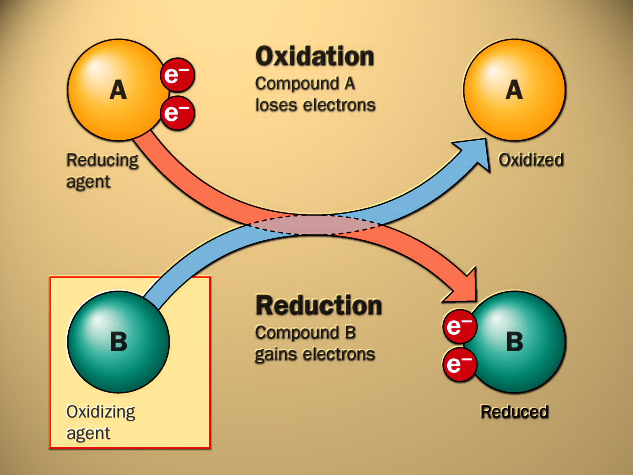
Half Equations
Half equations are the manner in which we describe the reactions taking place for the two compounds in question. An example might be as follows:
In this case, has donated an electron (has been oxidised) and has accepted the electrons (has been reduced). We can balance these equations (1) and (2) to obtain the full redox equation:
The E-Series
Not all reactions of this form will be spontaneous. For instance, reversing equations (1) and (2) would not yield a spontaneous reaction. The reason comes back to the relative stability and general reactivity of each atom compared to others. In order for a redox reaction to be spontaneous, we require that:
- The product of (1) be a weaker oxidant than the reactant of (2), or phrased differently
- The product of (2) to be a weaker reductant than the reactant of (1)
The ordering of oxidants and reductants is given in the E-series:
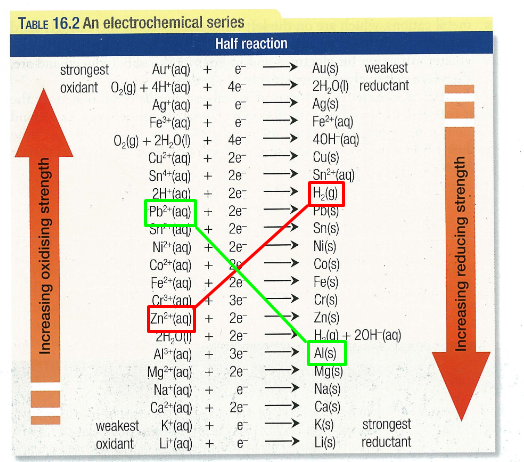
Competing Reactions
Suppose I give you a beaker with a calcium electrode and nickel sulfate. We have present: Ca(s), ions from the electrolyte but also since the solution is (presumed) aqueous. Observing the E-Series above, there are two viable redox reactions that can take place: both involving Ca as the reductant and either or as the oxidant. So which will take place?
Well, both may to an extent, but the one involving the nickel ions will dominate. Why? Because it is electrically the most favourable pathway. This is simply determined by the reation with the steepest “gradient” insofar as the lines I have drawn on the above figure. This fact of competition is extremely important when it comes to electrolytic cells.
Conclusion
The final point to note here is that if you put a redox reaction like the above-mentioned in the form of a galvanic cell, then you have a viable way of producing electricity (AKA voltage AKA a potential difference) via the spontaneous reaction.
Galvanic Cells
Contents
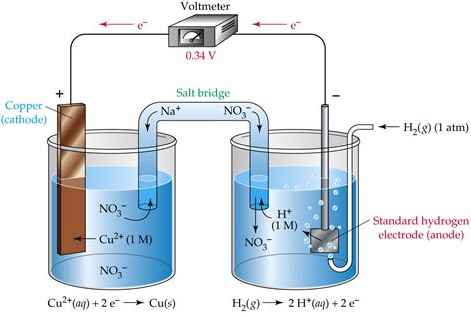
A galvanic cell is just a battery. It provides a way of converting chemical energy into electrical energy via a redox reaction. The cell consists of:
- An anode
- A cathode
- A salt bridge
- Some way of storing or using the charge supplied by the circuit
The amount of charge we get out of the cell is given by the values of the two species engaged in the redox reaction.
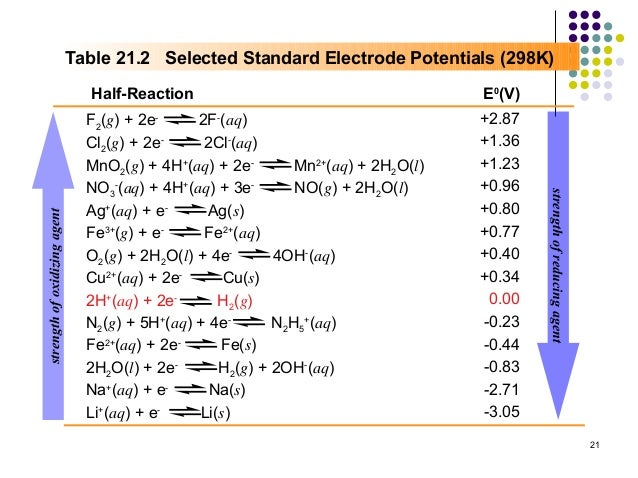
For instance, reducing along with oxidising would generate a voltage of V. This is theoretical only and experimental values would likely be slightly lower than this for a variety of factors (e.g. accruing electrode resistance).
Anode
The anode is the site of oxidation. In a galvanic cell, it is negatively charged. Intuitively, this makes sense. The anode releases electrons into the surrounding circuit and so will develop a negative charge overall. The electrons produced at the anode, however, will be attracted to the positive side of the cell (cathode).
Cathode
The cathode is the site of reduction. In a galvanic cell, it is positively charged. Again, because it is absorbing electrons, this should hopefully make sense.
Electrolyte
The electrolyte is some kind of ionic salt like potassium hydroxide of copper sulfate. In aqueous form, the two ions disassociate and are free to travel through the cell. The cations (positive ions, normally the metal ion) are attracted towards the anode, and the anions (negative ions) travel towards the cathode.
Salt Bridge
In order for a current to flow, an electrical circuit has to be connected. This is where the salt bridge comes in. It is usually just some membrane that allows for the ions in the electrolyte to migrate from one side of the cell to another. This allows current to flow and hence for a voltage to be produced.
Electrolysis
At base, electrolysis is the opposite of a galvanic cell. We supply a voltage in order to force a non-spontaneous reaction to occur. Instead of observing redox reactions like the green one (in the above figure), we observe the red. The voltage that needs to be supplied to the cell is at least the voltage specified by the difference in values of the two species we wish to reduce/oxidise.
Key differences between an electrolytic cell are that:
- The reaction taking place is non-spontaneous.
- This means the products formed must be kept separated, lest they react spontaneously to the original reactants upon contact
- The anode is positively charged (still the site of oxidation)
- The cathode is negatively charged (still the site of reduction)
Faraday’s Constant
We are able to easily predict the amount of a particular molecule/atom that will be reduced under the supplied voltage to the cell. For this example, consider the half-equation of .
Faraday’s constant is given by and represents the amount of charge carried per mole of electrons. Suppose I supply a current of for 60 seconds. Since current () is given by where is charge (in Coulombs) and is time, it follows that C. The relevant equation we now use to calculate the number of electrons that are transferred in the circuit for that charge is:
So, in our example, mol. But, from the half equation, for every 2 electrons, 2 atoms will oxidise. So mol as well.
This technique is very useful in Electrodeposition. If we want to depoist grams of copper onto an electrode, we can quite accurately the amount of current (and hence voltage) that we would need to supply to the circuit and for how long.
Competing Reactions
As with galvanic cells, we need to be careful considering what reactions will occur when three or more species are present in a particular environment.
Suppose I have the components for two (well-defined) redox reactions in an electrolytic cell, one requiring a voltage of and the other requiring a voltage of . There are two important observations to make:
- If I supply only, then only the first redox reaction will take place
- If I supply , then both redox reactions will take place in a particular ratio (which is difficult to predict, and depends largely on concentrations, temperature and pressure).
This is one of the challenges with CO2 Electroreduction: the cell potential required to reduce to products we want like ethylene is greater than the potential required for to reduce to other organic products.
Overpotentials
By definition, an overpotential is the potential difference between the half-reaction’s value and the potential at which the redox event is experimentally observed. It is a good measure of the cell’s efficiency. Clearly, you want the overpotential to be as small as possible, since you are otherwise wasting joules of energy that need not be used in the redox process.
Experimentally, it can be calculated by measuring the cell potential at which a given current density (typically a small one) is achieved. There are a number of different types of overpotentials:
- Activation overpotential - there is often a non-zero amount of energy that needs to be supplied in order to liberate/transfer an electron from electrode to electrolyte
- Reaction overpotential - this refers to the electrical energy wasted in driving non-preferential chemical reactions that precede the electron transfer. Refer to the above section for further details.
- Concentration overpotential - The depletion of charge-carriers (anions and cations in the electrolyte) at the electrode surface
- Bubble overpotential - the specific form of concentration overpotential related to the evolution of gas near the electrodes. This evolution reduces the effective area for the current transfer and thus increases current density.
- Resistance overpotential - the overpotentials related to the cell design, or inherent paths of resistance in the cell that increase the energy required to achieve redox.